01On the stage of BCMACar-T and TCE compete on the same stage
Since the beginning of this year, news related to BCMA has come and gone.
In February, the BCMA Car-T therapeutic product Carvykti, which legendary creatures cooperated with Johnson & Johnson, was approved by the FDA of the United States, representing the success of China’s original innovation. At the beginning of August, Amgen quietly revealed that it had terminated the development of AMG701, the last BCMA drug in the pipeline. From having two double antibodies and an ADC in this target, to clearing the warehouse now, the change was embarrassing. Just two weeks later, it was reported that Johnson & Johnson’s CD3xBCMA double antibody Tecvayli obtained the conditional listing license of the European Union.
Why is the target of BCMA so hot?
BCMA (B-cell maturation antigen) is mainly expressed on the surface of mature B lymphocytes and plasma cells. When plasma cells in bone marrow proliferate abnormally, it can lead to multiple bone injuries and gradually develop into Multiple Myeloma, MM). MM mainly occurs in the elderly over 65 years old and is the second most common haematoma in the United States. In the past, the incidence rate in China was relatively low, accounting for about 20% of that in the United States. However, in recent years, with the economic development, the rate of medical treatment has increased, and the population is aging.The incidence of MM in China continues to rise, with data showing that it has reached 40-45% in the United States..
At present, MM is still a disease that is difficult to cure. Most patients will relapse more than once, and the remission time will be shortened after each relapse, which makes great demands on the treatment of relapsed or refractory MM (R/RMM). The study found that,Overexpression and activation of BCMA exist in both newly diagnosed and relapsed patients, making BCMA a high potential target for R/R MM drug development.2。
Since the first BCMA drug was launched in 2020, four drugs targeting this target have been approved, coveringADC、Car-TandCD3 double antibodyThree types (Table 1).
Table 1: BCMA targeted drugs on the market
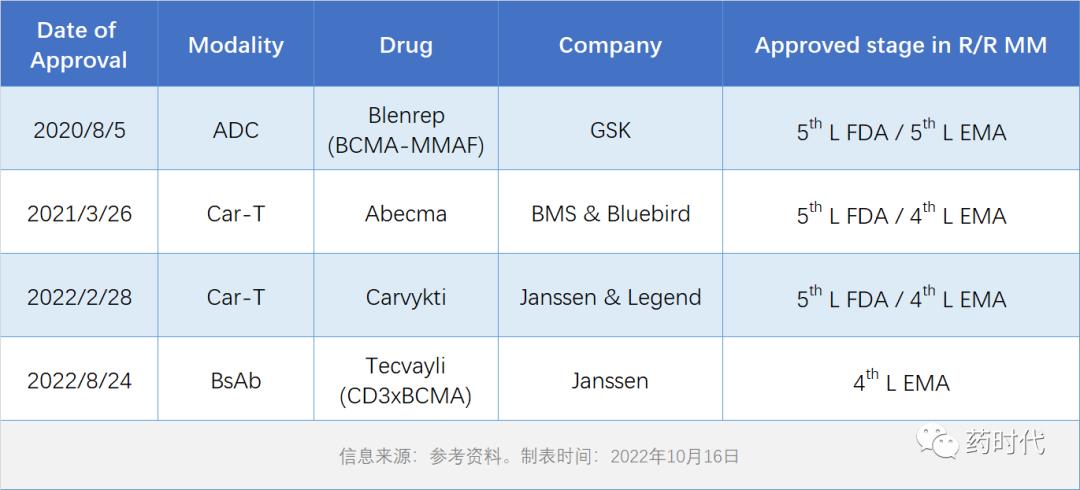
Although the indications are all 4-or 5-line treatment of R/R MM, it can be clearly seen from the clinical efficacy that the latecomers have made continuous breakthroughs compared with the pioneers.. Compared with the first Car-T product Abecma, the second Car-T product Carvykti has greatly improved its effectiveness (ORR, CR, DoR) and CRS safety. The newly approved double antibody Tecvayli, although its response rate (ORR, CR) is not as good as that of Carvykti, is already comparable to that of Abecma, and it is also closely followed by Carvykti on DoR. As a "spot" drug, it can be said that it is threatening (Table 2).
Table 2: Clinical effects of drugs listed in 2:BCMA
In fact, whether Car-T or CD3 double antibody, the mechanism of action is to bring killer T cells to tumor cells expressed by BCMA, and destroy tumor cells through the killing function of T cells. The difference is that Car-T can increase the number of T cells by in vitro amplification, while CD3 double antibody mobilizes the existing T cells in the body, soCar-T can be administered once, with strong and lasting effect.However, CD3 antibody is similar to traditional medicine and needs to be given regularly. But at the same time, unlike Car-T’s personalized and customized therapy,CD3 antibody is a spot product, which has natural advantages in convenience, accessibility and production cost.These are also the challenges that Car-T therapy needs to overcome to achieve universality.
02 CD3xBCMA molecule: Different platform, different fate.
The concept of Bispecific T-cell engagers (TCE) was actually verified as early as 2014 through the listing of Amgen Blincyto(CD3xCD19 BiTE).. However, there has been no other breakthrough in these years. Until this year, TCE ushered in spring: in January, Immunocore Kimmtrak, gp100xCD3 fusion protein, FDA approved melanoma; In June, Roche Lunsumio, CD20xCD3 double antibody, the European Union approved follicular lymphoma; In August, Johnson & Johnson’s Tecvayli, BCMAxCD3, and the EU approved R/R MM. In the middle of the eight years, TCE has cut through the thorns on the road ahead.
Amgen BiTE? vs Janssen DuoBody?
Although Amgen’s development of CD3xBCMA failed in the end, as a pioneer of FIC in this field, it brought valuable experience and value, which can be said that although it was defeated, it was still glorious. In 2012, Amgen acquired Micromet, the inventor of BiTE technology, and in 2016, it repurchased CD3xBCMA BiTE, namely AMG420, from BI. AMG420 is the first batch of BCMA double antibodies that entered the clinic in 2015, and the phase I showed good curative effect.The potential of the concept of CD3xBCMA is verified.. However, due to the toxicity, the maximum dose is 400 ug/day, and the half-life is short, so it needs to be administered every day. Then, Amgen developed AMG701 based on BITE-HLT (half-life extension) technology, which started clinical practice in 2017. Although the frequency of drug administration was improved to once a week, the project was completely terminated in August this year because of its comprehensive effectiveness and safety competitiveness. Looking at Johnson & Johnson/Jansen, in 2012, we chose to cooperate with Genmab’s DuoBody double antibody platform, from which we hatched Tecwayli (CD3XBCMA). Tecvayli started clinical practice in May 2017, and started all the way. At the end of 2021, according to the results of clinical phase I/II MajesTEC-1, it submitted a listing application to the FDA. In August this year, it took the lead in obtaining a conditional listing permit in the European Union. On the BiTE platform based on scFv skeleton, Amgen has harvested Blincyto, but the following projects are all invincible; With the IgG skeleton provided by DuoBody platform, in the past two years, two consecutive double antibody molecules, RybRevont (Amivantamab, cmetxegfr) and Tecvayli, have been approved only by the speed of clinical I/II data and become popular fried chickens.Some people say that the success or failure of the double-resistance lies in the platform, which is exaggerated, but its importance is beyond doubt..
The challenge of TCE development vs the ability of double anti-platform
For CD3 TCE, overcoming the compatibility of effectiveness and safety and realizing an operable treatment window is the key to success. The CD3 used in AMG420 and AMG701 has the same high affinity as Blincyto. With the compact and flexible skeleton of BiTE, a tight immune synapse is formed between tumor cells and T cells, which leads to the strong activation of T cell function and the release of cytokines at the same time of tumor killing, resulting in a narrow treatment window. At the same time, due to the small molecular weight (~55 kD) and short half-life, BiTE needs to be administered daily. On the other hand, Tecvayli uses CD3 with high affinity, but its binding kinetics has been adjusted with high probability, and it is constructed in IgG skeleton (150 kD), which is beneficial to optimize the activation effect of T cells, thus achieving safe and effective clinical performance and once-a-week administration frequency. At the same time, recent studies have found that the killing function of T cells can be activated specifically by reducing the affinity of CD3, so as to avoid the association with the release of cytokines, thus expanding the treatment window, such as Abbv-383B, which is in clinical stage I of Abbvie.
Fig. 1: The mechanism of 1:TCE, and the molecular form 2 of BiTE (A) and DuoBody (B) double antibody platforms, while the other arm of CD3 TCE double antibody binds to tumor surface antigen. For hematomas, targets such as BCMA and CD20 have specific expression profiles and are limited to B lymphocytes. This specificity helps to achieve safety and contributes to the first success of TCE in hematological tumors. For solid tumors, tumor-associated antigens are often low-expressed in normal tissues. How to selectively bind to tumor sites and avoid the on-target off-tumor effect on normal tissues is a big challenge to TCE.At present, a mainstream strategy is to design antigen binding with high titer and weak affinity by optimizing the titer and affinity of antigen recognition arm, so as to improve the tendency of tumor tissue with high antigen expression.. On the other hand, in solid tumors, TCE needs to break through the tumor microenvironment of immunosuppression and the physical barrier of tumor matrix, so drug combination will be the trend, which also requires TCE to have a better treatment window and safety. The design and consideration of TCE are quite complicated, 7,8, and this is just a superficial mention. But it can be seen,TCE molecules put forward higher requirements for the double antibody platform.It not only needs to meet the coexistence of two antigen regions of general double antibody, avoid mismatch and realize the feasibility and efficiency of quantitative production; It is also necessary to comprehensively coordinate the affinity, binding kinetics, valence ratio, spatial position and other considerations of the two antigen arms, and require the platform to support more flexible and diverse molecular design.
At present, there are several dual-antibody platforms with independent intellectual property rights in China, each of which has its own advantages in molecular composition.. From the point of view of flexibility, HBICE platform of Platinum Pharmaceuticals and WuXiBody and SDArBody platform of Yaoming Bio have more advantages, which can realize symmetrical and asymmetrical modes and different valence collocation of the two arms (Table 3). Youzhiyou complements each other through two platforms: YBody and CheckBody. At the same time, these companies have also made small achievements in the field of CD3 double antibodies, such as CD3xClaudin18.2 licensed by Platinum Medicine to AstraZeneca, three TCE projects by Youzhiyou, and TCE projects of several companies by WuXiBody platform, with the fastest progress in clinical phase I..
Table 3: Common Double Antibody Platforms in China
03 BCMA related pipelines and transactions Valuable battlefields will never lack players.
Internationally, Pfizer Elranatamab has entered clinical phase III, Regeneron REGN5458 is in clinical phase II, and Abbvie ABBV-383 & HPN-217 and BMS CC-93269 are in clinical phase I. In the field of BCMA, domestic enterprises have made faster progress in cell therapy than TCE, and there are more players, such as Abcema, which has been listed abroad, Iqalunsei (CT103A), which is a cooperation between Reindeer and Cinda, which is now under NMPA listing approval, and Zewo Kirensei (CT053), which is planned to be submitted to NDA at the end of this year. In the field of CD3xBCMA double antibody, although domestic enterprises have some layouts, they are all in a relatively early stage. At present, the fastest progress is the shore biological, clinical I/II phase in the United States and Australia; Approved clinical enterprises include Shandong New Age, Connoa and Zhixiang Jintai under Lunan Pharmaceutical; Preclinical projects have been disclosed, including Platinum Medicine and Tianguangshi (Table 4). Table 4: domestic CD3xBCMA double antibody research projects
In response to its broad market potential, transactions related to BCMA have also appeared frequently in recent years.(table 5) It is worth noting that MNC may consider the layout of different molecular types, such as small molecules, antibodies or cell therapy, in order to consolidate its own pipeline competitiveness in the MM field. For example, Johnson & Johnson has CD38 monoclonal antibody, BCMA Car-T and CD3xBCMA monoclonal antibody, as well as CD3xGPRC5D monoclonal antibody and CD3xGPRC5DxBCMA monoclonal antibody in the pipeline under research; Abbvi introduced two molecules, Abbv-383B and HPN217, to a target of CD3xBCMA. In the field of cell therapy, the focus is gradually expanding from autosomal Car-T to heteromorphic Car-T, such as P-BCMA-ALLO1 of Poseida introduced by Roche in August this year.
Table 5: Overview of authorized transactions involving BCMA targeted therapy in recent 3 years
04 Let a hundred flowers blossom, the future can be expected!
At present, Abecma, Carvykti and Tecvayli have the same number of approved lines in the EU, all of which are four-line treatment (Table 1). Although Car-T may be cured, according to the current data, most patients will still relapse. Moreover, the current autologous cell therapy requires patients to wait in a professional treatment center for several weeks before receiving treatment to prepare Car-T cells, which may not only lead to the deterioration of the disease and fall off during the period, but also fail to achieve treatment because the cell quality is not up to the requirements. Therefore, under the condition that the curative effect is not far different and the number of approved lines is the same,As a "spot" drug, TCE has obvious advantages in terms of drug accessibility, cost and patient compliance.. In the United States, Tecvayli is still under BLA review, and the number of approved lines in the future deserves our attention.Whether it is antibody drugs or Car-T, the frontline expansion of indications is a battleground for military strategists.. Comparing ADC and Car-T as five-line therapy, if Tecvayli can move forward, the competitive advantage will be more prominent. In this regard, Car-T therapy also needs to further examine the effect of BCMA drug-resistant patients. Although BCMA is the most common target in R/R MM research, TCE double antibodies targeting CD38, FcRH5 and GPRC5D are also being explored, and even two tumor surface antigens are simultaneously bound by three specific antibodies in order to further improve the response rate and sensitivity. Some research projects include Johnson & Johnson’s CD3xGPRC5D and CD3xGPRC5DxBCMA, Ichnos Sciences’ CD3xCD38 and CD3xCD38xBCMA, Roche’s CD3xFcRH5, and CD3xBCMAxPD-L1 of CDR-Life.
This is an era when a hundred flowers blossom, and the Eight Immortals cross the sea and show their magical powers. Perhaps in the near future, the treatment of multiple myeloma can be similar to the management of chronic diseases, and even finally achieve cure.
关于作者